 Image credit: G. Reddy
Image credit: G. Reddy
Abstract
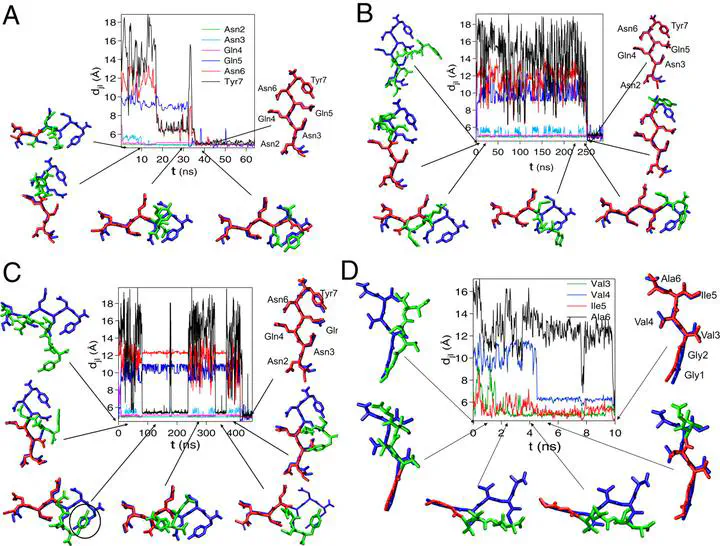
Sequence-dependent variations in the growth mechanism and stability of amyloid fibrils, which are implicated in a number of neurodegenerative diseases, are poorly understood. We have carried out extensive all-atom molecular dynamics simulations to monitor the structural changes that occur upon addition of random coil (RC) monomer fragments from the yeast prion Sup35 and Aβ-peptide onto a preformed fibril. Using the atomic resolution structures of the microcrystals as the starting points, we show that the RC → β-strand transition for the Sup35 fragment occurs abruptly over a very narrow time interval, whereas the acquisition of strand content is less dramatic for the hydrophobic-rich Aβ-peptide. Expulsion of water, resulting in the formation of a dry interface between 2 adjacent sheets of the Sup35 fibril, occurs in 2 stages. Ejection of a small number of discrete water molecules in the second stage follows a rapid decrease in the number of water molecules in the first stage. Stability of the Sup35 fibril is increased by a network of hydrogen bonds involving both backbone and side chains, whereas the marginal stability of the Aβ-fibrils is largely due to the formation of weak dispersion interaction between the hydrophobic side chains. The importance of the network of hydrogen bonds is further illustrated by mutational studies, which show that substitution of the Asn and Gln residues to Ala compromises the Sup35 fibril stability. Despite the similarity in the architecture of the amyloid fibrils, the growth mechanism and stability of the fibrils depend dramatically on the sequence.