Dry Amyloid Fibril Assembly in a Yeast Prion Peptide is Mediated by Long-Lived Structures Containing Water Wires
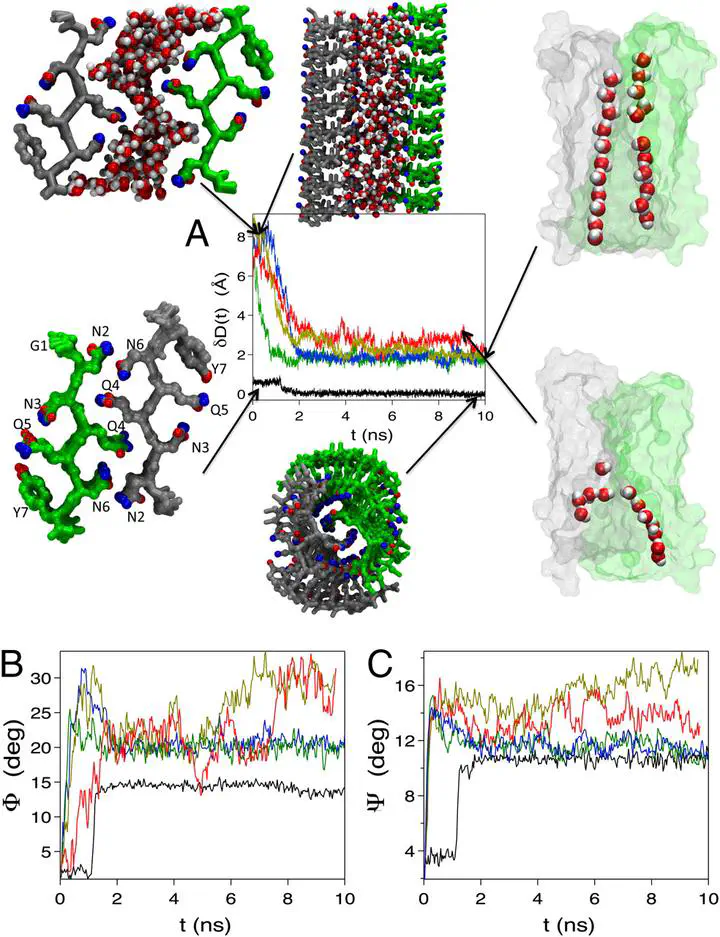 Image credit: G. Reddy
Image credit: G. Reddy
Abstract
Amyloid-like fibrils from a number of small peptides that are unrelated by sequence adopt a cross-β-spine in which the two sheets fully interdigitate to create a dry interface. Formation of such a dry interface is usually associated with self-assembly of extended hydrophobic surfaces. Here we investigate how a dry interface is created in the process of protofilament formation in vastly different sequences using two amyloidogenic peptides, one a polar sequence from the N terminus of the yeast prion Sup35 and the other a predominantly hydrophobic sequence from the C terminus of Aβ-peptide. Using molecular dynamics simulations with three force fields we show that spontaneous formation of two ordered one-dimensional water wires in the pore between the two sheets of the Sup35 protofilaments results in long-lived structures, which are stabilized by a network of hydrogen bonds between the water molecules in the wires and the polar side chains in the β-sheet. Upon decreasing the stability of the metastable structures, water molecules are expelled resulting in a helically twisted protofilament in which side chains from a pair of β-strands in each sheet pack perfectly resulting in a dry interface. Although drying in hydrophobically dominated interfaces is abrupt, resembling a liquid to vapor transition, we find that discrete transitions between the liquid to one-dimensional ordered water in the nanopore enclosed by the two β-sheets to dry interface formation characterizes protofilament assembly in the yeast prions. Indeed, as the two sheets of the hydrophobic Aβ-sequence approach each other, fibril formation and expulsion of water molecules occur rapidly and nearly simultaneously.