 Image credit: G. Reddy
Image credit: G. Reddy
Abstract
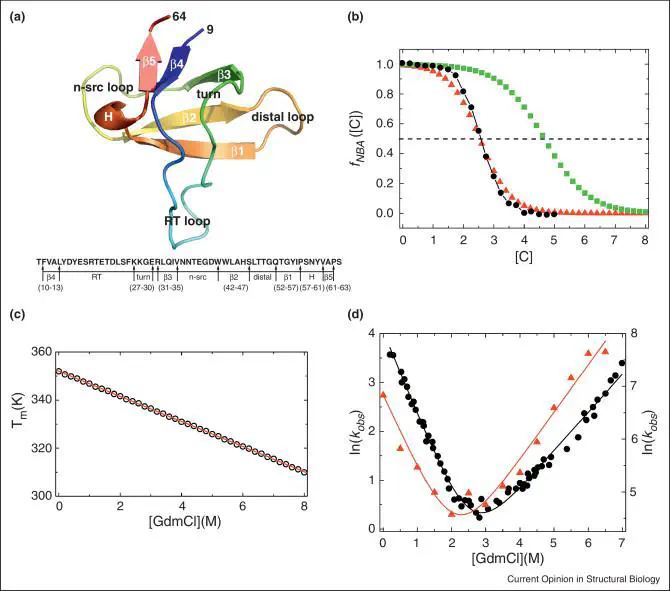
A quantitative theory of protein folding should make testable predictions using theoretical models and simulations performed under conditions that closely mimic those used in experiments. Typically, in laboratory experiments folding or unfolding is initiated using denaturants or external mechanical force, whereas theories and simulations use temperature as the control parameter, thus making it difficult to make direct comparisons with experiments. The molecular transfer model (MTM), which incorporates environmental changes using measured quantities in molecular simulations, overcomes these difficulties. Predictions of the folding thermodynamics and kinetics of a number of proteins using MTM simulations are in remarkable agreement with experiments. The MTM and all atom simulations demonstrating the presence of dry globules represent major advances in the proteins folding field.