 Image credit: G. Reddy
Image credit: G. Reddy
Abstract
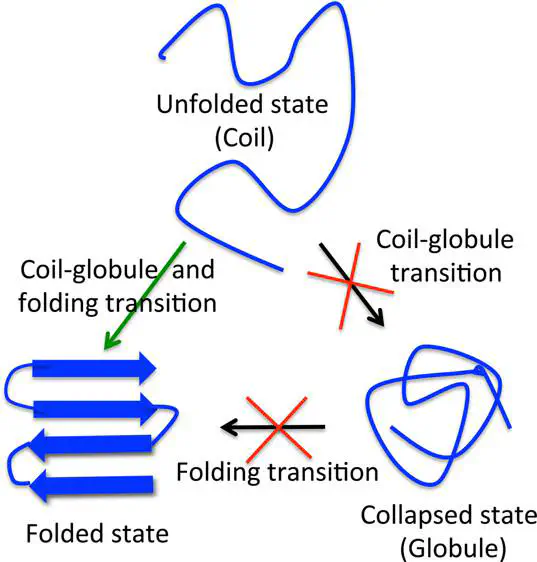
A fundamental question in protein folding is whether the coil to globule collapse transition occurs during the initial stages of folding (burst phase) or simultaneously with the protein folding transition. Single molecule fluorescence resonance energy transfer (FRET) and small-angle X-ray scattering (SAXS) experiments disagree on whether Protein L collapse transition occurs during the burst phase of folding. We study Protein L folding using a coarse-grained model and molecular dynamics simulations. The collapse transition in Protein L is found to be concomitant with the folding transition. In the burst phase of folding, we find that FRET experiments overestimate radius of gyration, Rg, of the protein due to the application of Gaussian polymer chain end-to-end distribution to extract Rg from the FRET efficiency. FRET experiments estimate ≈6 Å decrease in Rg when the actual decrease is ≈3 Å on guanidinium chloride denaturant dilution from 7.5 to 1 M, thereby suggesting pronounced compaction in the protein dimensions in the burst phase. The ≈3 Å decrease is close to the statistical uncertainties of the Rg data measured from SAXS experiments, which suggest no compaction, leading to a disagreement with the FRET experiments. The transition-state ensemble (TSE) structures in Protein L folding are globular and extensive in agreement with the Ψ-analysis experiments. The results support the hypothesis that the TSE of single domain proteins depends on protein topology and is not stabilized by local interactions alone.