 Image credit: G. Reddy
Image credit: G. Reddy
Abstract
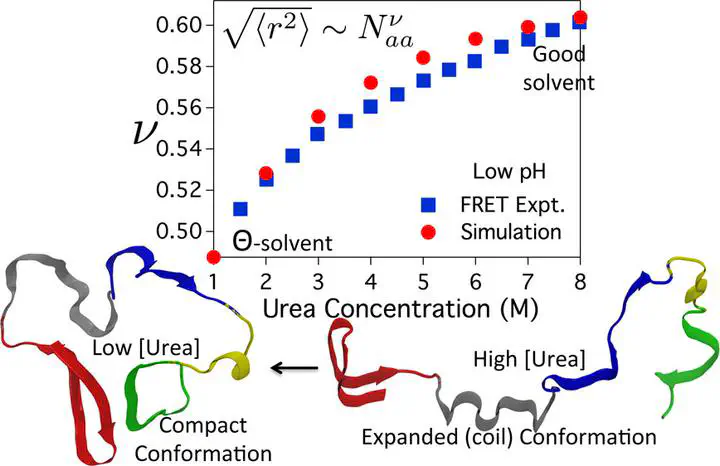
The folding of small protein ubiquitin (Ub), which plays an indispensable role in targeting proteins for degradation and DNA damage response, is complex. A number of experiments on Ub folding have reached differing conclusions regarding the relation between collapse and folding, and whether intermediates are populated. In order to resolve these vexing issues, we elucidate the denaturant-dependent thermodynamics and kinetics of Ub folding at low and neutral pH as a function of guanidinium chloride and urea using coarse-grained molecular simulations. The changes in the fraction of the folded Ub, and the radius of gyration (Rg) as a function of the denaturant concentration, [C], are in quantitative agreement with experiments. Under conditions used in experiments, Rg of the unfolded state at neutral pH changes only by ≈17% as the [GdmCl] decreases from 6 to 0 M. We predict that the extent of compaction of the unfolded state increases as temperature decreases. A two-dimensional folding landscape as a function of Rg and a measure of similarity to the folded state reveals unambiguously that the native state assembly is preceded by collapse, as discovered in fast mixing experiments on several proteins. Analyses of the folding trajectories, under mildly denaturing conditions ([GdmCl] = 1.0 M or [Urea] = 1.0 M), shows that Ub folds by collision between preformed secondary structural elements involving kinetic intermediates that are primarily stabilized by long-range contacts. Our work explains the results of small angle X-ray scattering (SAXS) experiments on Ub quantitatively, and establishes that evolved globular proteins in the unfolded ensemble are poised to collapse as the solvent conditions for the biopolymer changes from good solvent to Θ-solvent like conditions on denaturant dilution. In the process, we explain the discrepancy between SAXS and single molecule fluorescent resonant energy transfer (smFRET) experiments, which have arrived at a contradicting conclusion concerning the collapse of polypeptide chains.