 Image credit: G. Reddy
Image credit: G. Reddy
Abstract
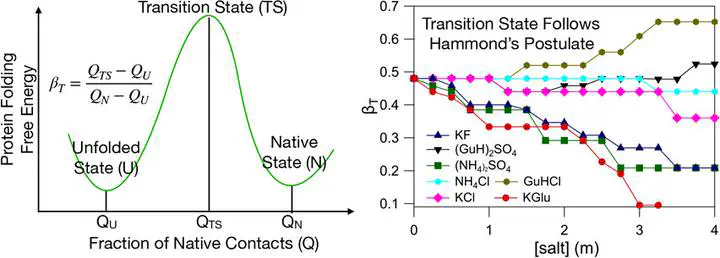
Salts differ in their ability to stabilize protein conformations, thereby affecting the thermodynamics and kinetics of protein folding. We developed a coarse-grained protein model that can predict salt-induced changes in protein properties by using the transfer free-energy data of various chemical groups from water to salt solutions. Using this model and molecular dynamics simulations, we probed the effect of seven different salts on the folding thermodynamics of the DNA binding domain of lac repressor protein (lac-DBD) and N-terminal domain of ribosomal protein (NTL9). We show that a salt can act as a protein stabilizing or destabilizing agent depending on the protein sequence and folded state topology. The computed thermodynamic properties, especially the m values for various salts, which reveal the relative ability of a salt to stabilize the protein folded state, are in quantitative agreement with the experimentally measured values. The computations show that the degree of protein compaction in the denatured ensemble strongly depends on the salt identity, and for the same variation in salt concentration, the compaction in the protein dimensions varies from ∼4% to ∼30% depending on the salt. The transition-state ensemble (TSE) of lac-DBD is homogeneous and polarized, while the TSE of NTL9 is heterogeneous and diffusive. Salts induce subtle structural changes in the TSE that are in agreement with Hammond’s postulate. The barrier to protein folding tends to disappear in the presence of moderate concentrations (∼3–4 m) of strongly stabilizing salts.