 Image credit: G. Reddy
Image credit: G. Reddy
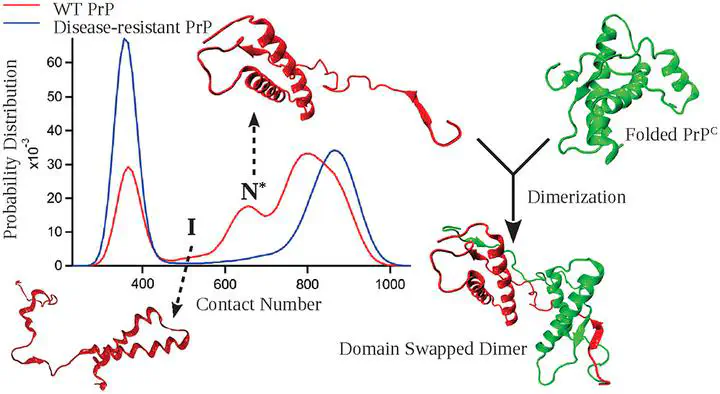
Abstract
Theory and simulations predicted that the sizes of the unfolded states of globular proteins should decrease as the denaturant concentration is reduced from a high to a low value. However, small angle X-ray scattering (SAXS) data were used to assert the opposite, while interpretation of single molecule Förster resonance energy transfer experiments (FRET) supported the theoretical predictions. The disagreement between the two experiments is the SAXS-FRET controversy. By harnessing recent advances in SAXS and FRET experiments and setting these findings in the context of a general theory and simulations, which do not rely on experimental data, we establish that compaction of unfolded states under native conditions is universal. The theory also predicts that proteins rich in β-sheets are more collapsible than α-helical proteins. Because the extent of compaction is small, experiments have to be accurate and their interpretations should be as model-free as possible. Theory also suggests that collapsibility itself could be a physical restriction on the evolution of foldable sequences, and also provides a physical basis for the origin of multidomain proteins.