Local RNA Structure, Ion Hydration Shell and the Energy Barrier for Water Exchange from the Ion Hydration Shell Determine the Mechanism of Ion Condensation on Specific RNA Sites
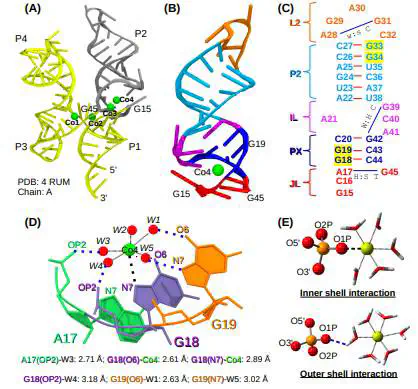 Image credit: G. Reddy
Image credit: G. Reddy
Abstract
RNA folding and functioning require the binding of metal ions in specific cavities of the folded structure. This property is critical to the functioning of riboswitches that especially regulate the metal ions concentration in bacteria. However, the fundamental principles governing the specific binding of metal ions in RNA are unclear. We probed the condensation mechanism of biologically relevant alkali (Na+ and K+), alkaline earth (Mg2+ and Ca2+), and transition metals (Mn2+, Co2+, Ni2+ and Zn2+) on a part of the Ni2+ and Co2+ (NiCo) sensing riboswitch aptamer domain using computer simulations. The selected structure has multiple secondary structural elements and a single site for the specific binding of a metal ion. We show that three factors primarily determine the binding of a metal ion to an RNA site - (1) The varying structural constraints from different RNA secondary structural elements strongly influence the metal ion binding. The mode of ion binding depends on the local structure around the RNA’s ion-binding pocket. (2) The arrangement of water molecules in the ion hydration shell, and (3) the energy barrier for the ion to lose a water molecule from its hydration shell and transition from an outer to an inner shell interaction, which is primarily influenced by the metal ion charge density. These results have implications for designing biocompatible sensors using riboswitches to probe the concentration of intracellular metal ions.